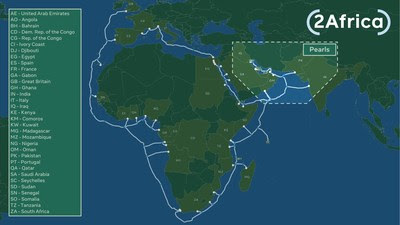
Now the Longest Subsea Cable System in the World
MENLO PARK, Calif., Sept. 28, 2021 /PRNewswire/ — The 2Africa consortium, comprised of China Mobile International, Facebook, MTN GlobalConnect, Orange, stc, Telecom Egypt, Vodafone and WIOCC, announced today the addition of a new segment – the 2Africa PEARLS branch – extending to the Arabian Gulf, India, and Pakistan. This extension will bring the total length of the 2Africa cable system to over 45,000 kilometers, making it the longest subsea cable system ever deployed.
Now connecting three continents, Africa, Europe and Asia terrestrially through Egypt, 2Africa creates unique connectivity by adding vital landing locations in Oman (Barka), UAE (Abu Dhabi and Kalba), Qatar (Doha), Bahrain (Manama), Kuwait (Kuwait), Iraq (Al-Faw), Pakistan (Karachi), India (Mumbai), and a fourth landing in Saudi Arabia (Al Khobar). The new 2Africa branch joins recently announced extensions to the Canary Islands, the Seychelles, Comoros Islands, Angola, and a new landing to south-east Nigeria.
As with other 2Africa cable landings, capacity will be available in PEARLS landings at carrier-neutral facilities or open-access cable landing stations on a fair and equitable basis, encouraging and supporting the development of a healthy internet ecosystem.
To further support a burgeoning global digital economy, the expanded system will serve an even wider range of communities that rely on the internet for services from education to healthcare, and businesses, providing economic and social benefits that come from increased connectivity. As announced in May 2020, 2Africa was planned to directly bring seamless international connectivity to 1.2 billion people. Today, with 2Africa PEARLS, 2Africa will be providing international connectivity to an additional 1.8 billion people–that’s 3 billion people, representing 36% of the global population.
Alcatel Submarine Networks (ASN) will deploy the new system utilizing new technologies such as SDM that allow the deployment of up to 16 fiber pairs, double that of older technologies and bringing greater and more cost-effective capacity.
About China Mobile International Limited
China Mobile International Limited (CMI) is a wholly-owned subsidiary of China Mobile, mainly responsible for the operation of China Mobile’s international business. In order to provide better services to meet the growing demand in the international telecommunications market, China Mobile established a subsidiary, CMI, in December 2010. CMI currently has over 70 terrestrial and submarine cable resources worldwide, with a total international transmission bandwidth of 98T, and more than 180 PoPs. With Hong Kong, China as its launchpad, CMI has significantly accelerated global IDC development, creating a strong network for data centre cloudification.
Leveraging the strong support by China Mobile, CMI is a trusted partner that provides comprehensive international information services and solutions to international enterprises, carriers and mobile users. Headquartered in Hong Kong, China, CMI has expanded its footprint in 37 countries and regions. For more information, please visit www.cmi.chinamobile.com.
About Facebook
Founded in 2004, Facebook’s mission is to give people the power to build community and bring the world closer together. Over 2 billion people use Facebook, Instagram, WhatsApp, or Messenger every month to stay connected with friends and family, to discover what is going on in the world, and to share and express what matters to them. Facebook is defined by its unique culture – one that rewards impact. The company encourages people to be bold and solve the problems they care most about. The phrase “this journey is 1% finished” reminds the company’s teams that they have only begun to fulfill Facebook’s mission. As the company evolves its journey to bring the world closer together, it stays true to the same core values to guide the way it works and the decisions it makes every step of the way: be open, be bold, move fast, focus on impact, and build social value. For more information, please visit https://www.linkedin.com/company/facebook/about/.
About MTN GlobalConnect
GlobalConnect is a Pan-African digital wholesale and infrastructure services company, and an operating company in the MTN Group. GlobalConnect manages MTN’s international and national major wholesale activities, in addition to offering reliable wholesale and infrastructure solutions for fixed connectivity and wholesale mobility solutions that include international mobile services, Voice, SMS, signalling, roaming and interconnect. The MTN Group launched in 1994 is a leading emerging market operator with a clear vision to lead the delivery of a bold new digital world and is inspired by the belief that everyone deserves the benefits of a modern connected life. Embracing the Ambition 2025 strategy, MTN is anchored on building the largest and most valuable platform business, with a clear focus on Africa. The MTN Group is listed on the JSE Securities Exchange in South Africa under the share code “MTN”.
For more information, please visit www.globalconnect.solutions – https://www.mtn.com
About Orange
Orange is one of the world’s leading telecommunications operators with sales of 42.3 billion euros in 2020 and 139,000 employees worldwide at 30 June 2021, including 80,000 employees in France. The Group has a total customer base of 263 million customers worldwide at 30 June 2021, including 218 million mobile customers and 22 million fixed broadband customers. The Group is present in 26 countries. Orange is also a leading provider of global IT and telecommunication services to multinational companies, under the brand Orange Business Services. In December 2019, the Group presented its new “Engage 2025” strategic plan, which, guided by social and environmental accountability, aims to reinvent its operator model. While accelerating in growth areas and placing data and AI at the heart of its innovation model, the Group will be an attractive and responsible employer, adapted to emerging professions.
Orange is listed on Euronext Paris (symbol ORA) and on the New York Stock Exchange (symbol ORAN).
For more information on the internet and on your mobile: www.orange.com, www.orange-business.com or to follow us on Twitter: @orangegrouppr.
About stc
With its headquarter in Riyadh, stc group is the largest in the Middle East and North Africa based on market cap. stc’s revenue for 2020 amounted to 58,953million SAR (15,721 million US dollars) and the net profit amounted to 10,995 million SAR (2,932 million US dollars). stc was established in 1998 and currently has customers around the globe. It is ranking among the world’s top 50 digital companies and the first in the Middle East and North Africa according to Forbes. It focuses on providing services to enterprise and consumer customers through a fiber-optic network that spans 217,000 kilometers. stc group was among the first in MENA region to launch 5G networks and was considered one of the fastest globally in deploying 5G network as stc already deployed around 4,000 5G towers as end of 2020. stc group has 14 subsidiaries in the Kingdom, gulf and around the world, and its own 100% of stc Bahrain, 51.8% stake in stc Kuwait and 25% stake in Binariang GSM Holding in Malaysia which owns 62% of Maxis in Malaysia.
In Saudi Arabia (the group’s main operation site) stc operates the largest modern mobile network in the Middle East as it covers more than 99% of the country’s populated areas in addition to providing 4G mobile broadband to about 90% of the population across the Kingdom of Saudi Arabia. In addition to the above-mentioned, stc is a strong regional player in IoT, managed services, system integration, cloud computing, information security, big data Analytics fintech and artificial intelligence. For more information, please visit https://www.stc.com.sa; or to follow us on Twitter: @stc , @stc_ksa
About Telecom Egypt
Telecom Egypt is the first total telecom operator in Egypt providing all telecom services to its customers including fixed and mobile voice and data services. Telecom Egypt has a long history serving Egyptian customers for over 160 years maintaining a leadership position in the Egyptian telecom market by offering its enterprise and consumer customers the most advanced technology, reliable infrastructure solutions and the widest network of submarine cables. Aside from its mobile operation “WE”, the company owns a 45% stake in Vodafone Egypt. Telecom Egypt’s shares and GDRs (Ticker: ETEL.CA; TEEG.LN) are traded on The Egyptian Exchange and the London Stock Exchange. Please refer to Telecom Egypt’s full financial disclosure on ir.te.eg
For more information, contact:
The investor relations team
Email: investor.relations@te.eg
About Vodafone
Vodafone is a leading telecommunications company in Europe and Africa. Our purpose is to “connect for a better future” enabling an inclusive and sustainable digital society. Our expertise and scale give us a unique opportunity to drive positive change for society. Our networks keep family, friends, businesses and governments connected and – as COVID-19 has clearly demonstrated – we play a vital role in keeping economies running and the functioning of critical sectors like education and healthcare.
Vodafone is the largest mobile and fixed network operator in Europe and a leading global IoT connectivity provider. Our M-Pesa technology platform in Africa enables 50m people to benefit from access to mobile payments and financial services. We operate mobile and fixed networks in 21 countries and partner with mobile networks in 49 more. As of 30 June 2021, we had over 300m mobile customers, more than 28m fixed broadband customers, over 22m TV customers and we connected 130m IoT devices.
We support diversity and inclusion through our maternity and parental leave policies, empowering women through connectivity and improving access to education and digital skills for women, girls, and society at large. We are respectful of all individuals, irrespective of race, ethnicity, disability, age, sexual orientation, gender identity, belief, culture or religion.
Vodafone is also taking significant steps to reduce our impact on our planet by reducing our greenhouse gas emissions by 50% by 2025 and becoming net zero by 2040, purchasing 100% of our electricity from renewable sources in Europe and across our entire operations by 2025 and reusing, reselling or recycling 100% of our redundant network equipment.
For more information, please visit www.vodafone.com, follow us on Twitter at @VodafoneGroup or connect with us on LinkedIn at www.linkedin.com/company/vodafone.
About WIOCC
WIOCC is building Africa’s first, truly hyper-scale network infrastructure. With the ability to efficiently deliver 100Gbps capacity and an extensive investment programme to develop our pan-African solution even further, WIOCC is the natural partner for OTTs, content providers, telecom operators, and ISPs looking to take advantage of Africa’s opportunities. The company utilises more than 55,000km of terrestrial fibre and in excess of 75,000km of submarine cable assets to offer affordable, reliable, managed connectivity to over 550 locations across 30 African countries. WIOCC’s international reach extends to key commercial centres in Europe, Asia, and North America, providing a one-stop shop for fully-scalable international connectivity into, within, and out of Africa. Operating exclusively as a wholesaler, the company’s focus is on putting you, our client, first. Building and maintaining strong, long-term relationships means WIOCC can develop bespoke solutions to meet your current requirements, with the flexibility to match future demands for growth and extra resilience and geographical expansion. You will find that only WIOCC has the depth of experience, local expertise, capacity, flexibility, and scalability to take you where you want to be. For more information, please visit http://wiocc.net/.
About Alcatel Submarine Networks
Alcatel Submarine Networks, part of Nokia, leads the industry in terms of transmission capacity and installed base with more than 650,000 km of optical submarine systems deployed worldwide, enough to circumnavigate the globe 15 times. From traditional Telecom applications to Content and “Over The Top” Service Provider infrastructures, as well as to offshore Oil and Gas applications, ASN provides all elements of a turnkey global undersea transmission systems, tailored to individual customer’s needs. An extensive Services portfolio completes its comprehensive offering for the submarine business, including project management, installation and commissioning, along with marine and maintenance operations performed by ASN’s fully owned fleet of cable ships. For more information, please visit https://web.asn.com/en/.
Photo – https://mma.prnewswire.com/media/1635450/2Africa___Pearls_Map_Updated.jpg